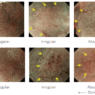
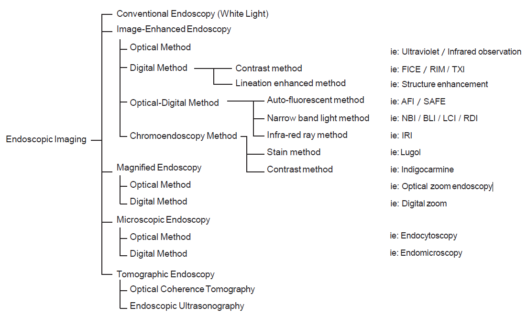
Squamous Cell Carcinoma
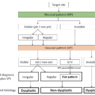
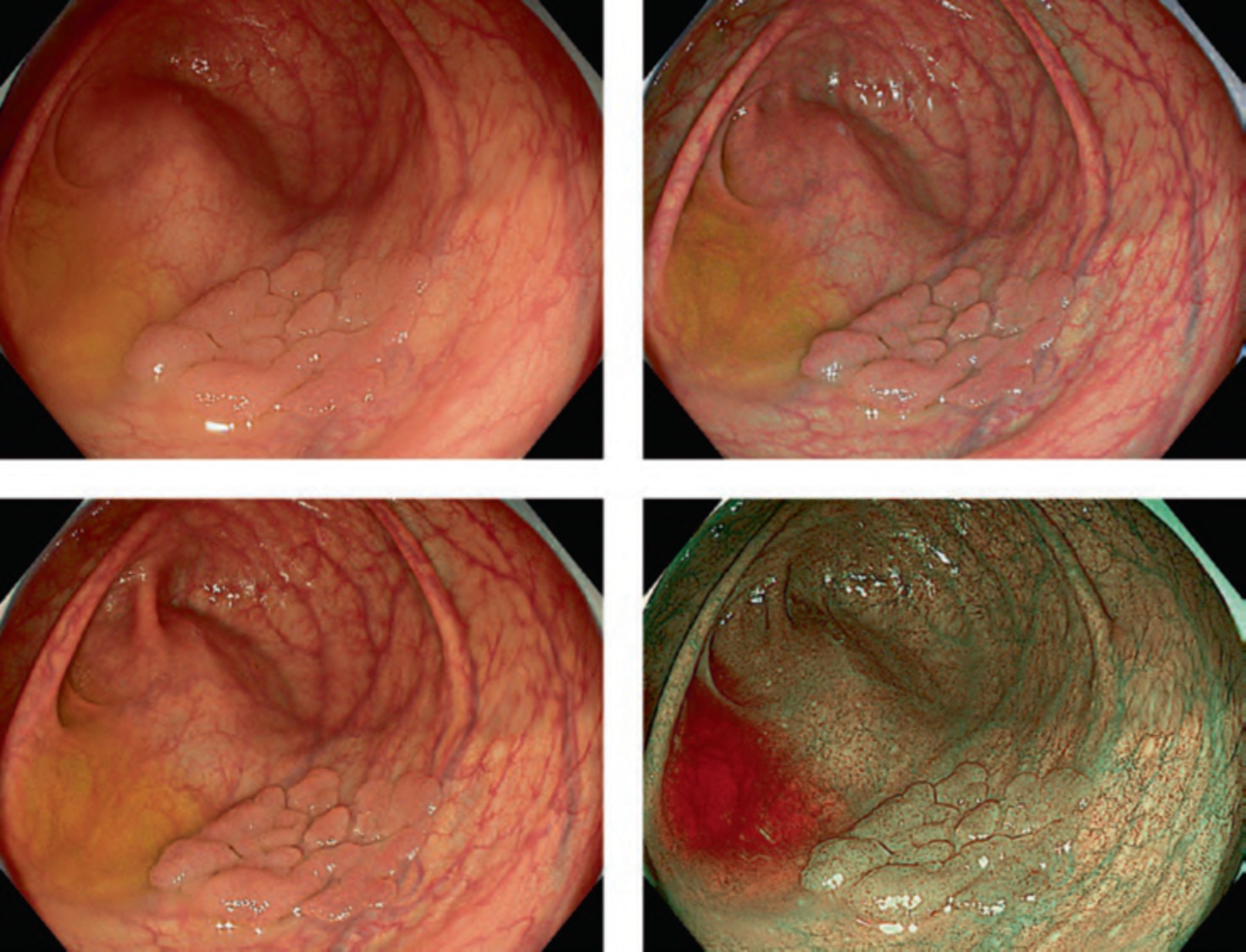
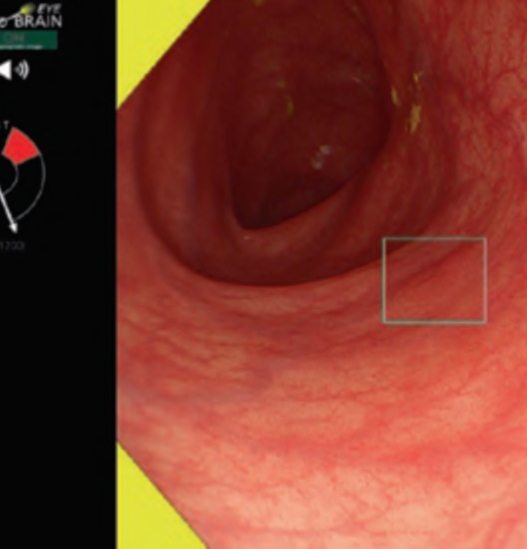
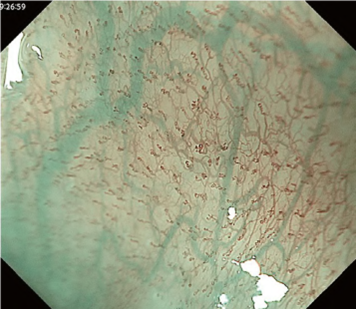
Predicting invasion depth of superficial esophageal squamous cell carcinoma is crucial in determining the precise indication for endoscopic resection (ER) because the rate of lymph node metastasis increases in proportion to the invasion depth of the carcinoma. Previous studies have shown a close relationship between microvascular patterns observed by magnifying endoscopy and invasion depth of the superficial carcinoma. Although there were two major classifications, Inoue and Arima, the Japan Esophageal Society (JES) integrated the two classifications and simplified it and developed a new magnifying endoscopic classification for the characterization and predicting invasion depth of superficial esophageal squamous cell carcinomas (SESCCs). This is essential for developing a treatment strategy for SESCC, in particular the indication for ER. Therefore, in this classification, morphological types of microvessels are classified into two categories of noncancerous [type A: normal epithelium, inflammation, and squamous intraepithelial neoplasia (SIN)] and cancerous (Type B: SCC) lesions. The cancerous types of microvessels corresponding to SESCCs are subclassified into three groups based upon an indication for ER as follows: an absolute indication type (Type B1: T1a-EP or T1a-LPM), a relative indication type [Type B2: T1a-MM or T1b-SM1(tumor invades the submucosa to a depth of 200 μm or less from the muscularis mucosa)], and a contraindication type [Type B3: T1b-SM2 (tumor invades the submucosa to a depth more than 200 μm)]. Diagnostic criteria of the JES classification are based on the degree of microvascular irregularity in the target lesion observed by magnifying endoscopy. Intrapapillary capillary loops (IPCL) are a basic unit of microvasculature in the stratified squamous epithelial layer. The microvascular irregularity is evaluated for the presence or absence of each of the following morphological factors: weaving (i.e., tortuosity), dilatation, irregular caliber, and different shape (i.e., various shapes). Microvessels are classified as type A if they have three or fewer factors (i.e., without severe abnormality; . Fig. 1a) and type B if they have all four (i.e., with severe abnormality). Type B is then subclassified into B1, B2, and B3 (. Fig. 1b–d, respectively) based on the running pattern or degree of dilatation of severely irregular microvessels. The definitions and schemas of type A and B vessels and predicted histology of invasion depth by type B vessels are summarized in . Table 1. A large scale validation study showed high overall accuracy (90.5%) of type B vessels of the JES classification. The most important auxiliary criterion in the JES classification is avascular area (AVA). AVA is defined as a low or no vascularity area surrounded by all subtypes of type B microvessels including B1 vessels. Diameters of AVA are positively correlated with sizes of histological cancer nest and the histological invasion depth of SESCC. Small (<0.5 mm), middle (0.5 ≤< 3 mm), and large (≤3 mm) AVA are suggestive of T1a-EP/ LPM, T1a-MM/T1b-SM1, and T1b-SM2, respectively. A key point to note is that any types of AVA (small, middle, and large) surrounded by B1 vessels are suggestive of T1a-EP or T1a-LPM SCC.
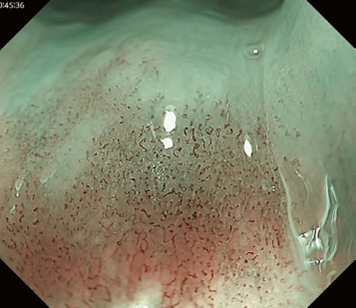
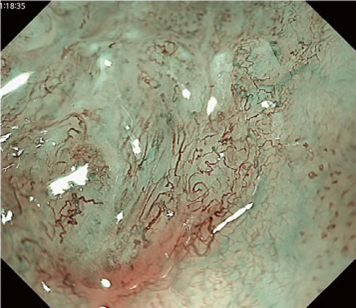
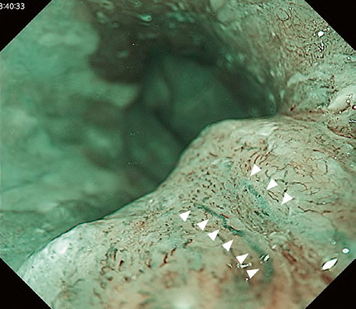
A meta-analysis comparing the Inoue, Arima, and JES classifications in the diag-nostic accuracy and staging of SESCC showed that diagnostic accuracy values of the three classifications for epithelial/lamina propria mucosa (EP/LPM) tumor staging were all high (Inoue, 87.2%; Arima, 98.7%; and JES, 86.7%), but for MM or SM1, the diagnostic accuracy of the JES was significantly higher than the Inoue classification (75.5% vs. 58.7%, P < 0.05), and for EP/LPM and SM2 or deeper, the diagnostic accuracy of the Arima classification was significantly higher than that of the other two (P < 0.01). However, diagnostic reproducibility (κ value) of the Arima classification was lower than that of the JES classification. The meta-analysis suggested that the JES classification will have high validity and reliability in the diagnosis of SESCC.
Barrett’s Esophagus (BE) and BE-Related Neoplasia
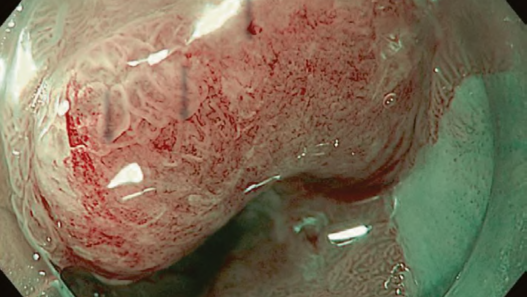
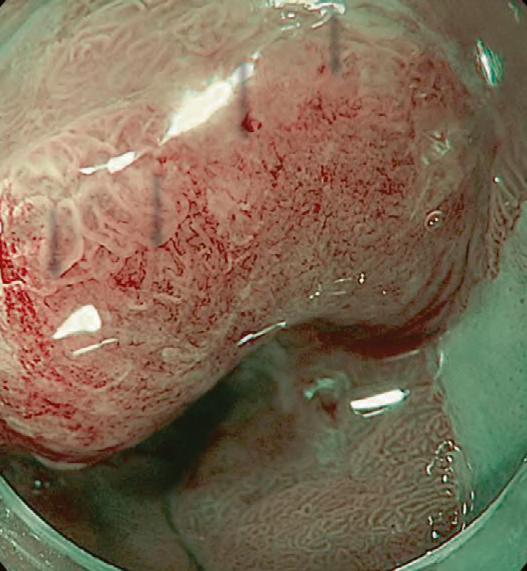
Studies showed an excellent prognosis among patients with superficial BE-related neoplasms (SBERN), including dysplasia and esophageal adenocarcinoma (EAC) confined to the submucosal layer. Early detection is the primary key factor for a favorable prognosis among patients with EAC. SBERN, especially the flat macroscopic type, is often difficult to detect by standard white-light endoscopy. Current guidelines in the West recommend endoscopic surveillance for patients with BE using random four-quadrant biopsies, in which samples are obtained based on the Seattle protocol to detect SBERN. However, the Seattle protocol is labor-intensive, time-consuming, expensive, and additionally associated with a high risk of sampling error.
A recent meta-analysis suggested that acetic acid enhancement, narrowband imaging (NBI), and endoscopy based confocal laser endomicroscopy are promising techniques for targeted biopsy that could be employed to eliminate the need for random biopsies. Several classifications using NBI magnifying endoscopy (NBI-M) have been proposed, thereby suggesting the high utility of the classifications for the diagnosis of SBERN. However, none of them have been widely accepted; thus, recent studies attempted to integrate and simplify the magnifying endoscopic classifications.
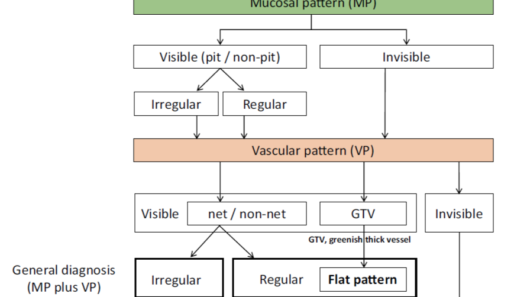
The Japan Esophageal Society Barrett’s Esophagus (JES-BE) working group has developed and proposed a new magnifying endoscopic classification system, including a diagnostic flowchart (. Fig. 2), to identify SBERN, namely, the JES-BE classification. The classification has three characteristics, simplified, an easily understood classification by incorporating well-known diagnostic criteria for early gastric cancer and modified criteria for a flat pattern.
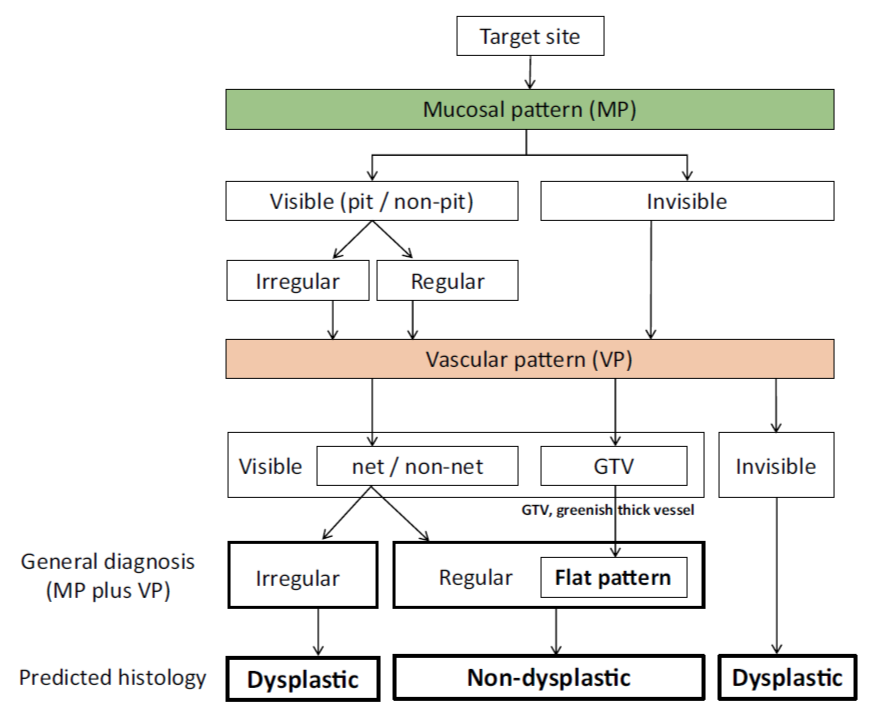
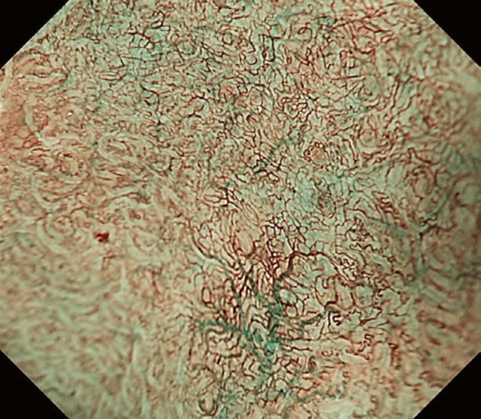
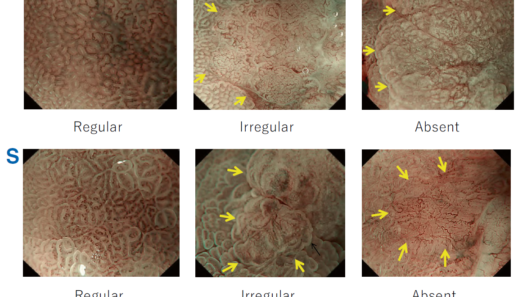
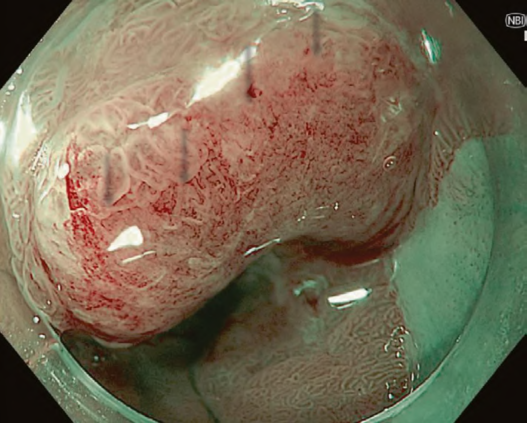
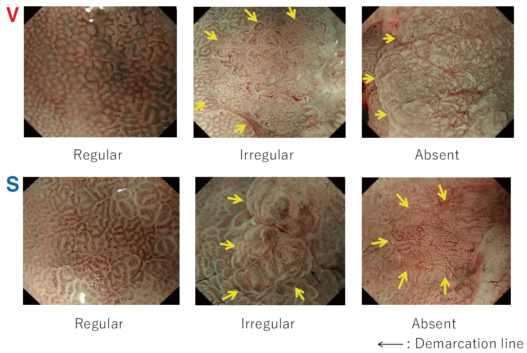
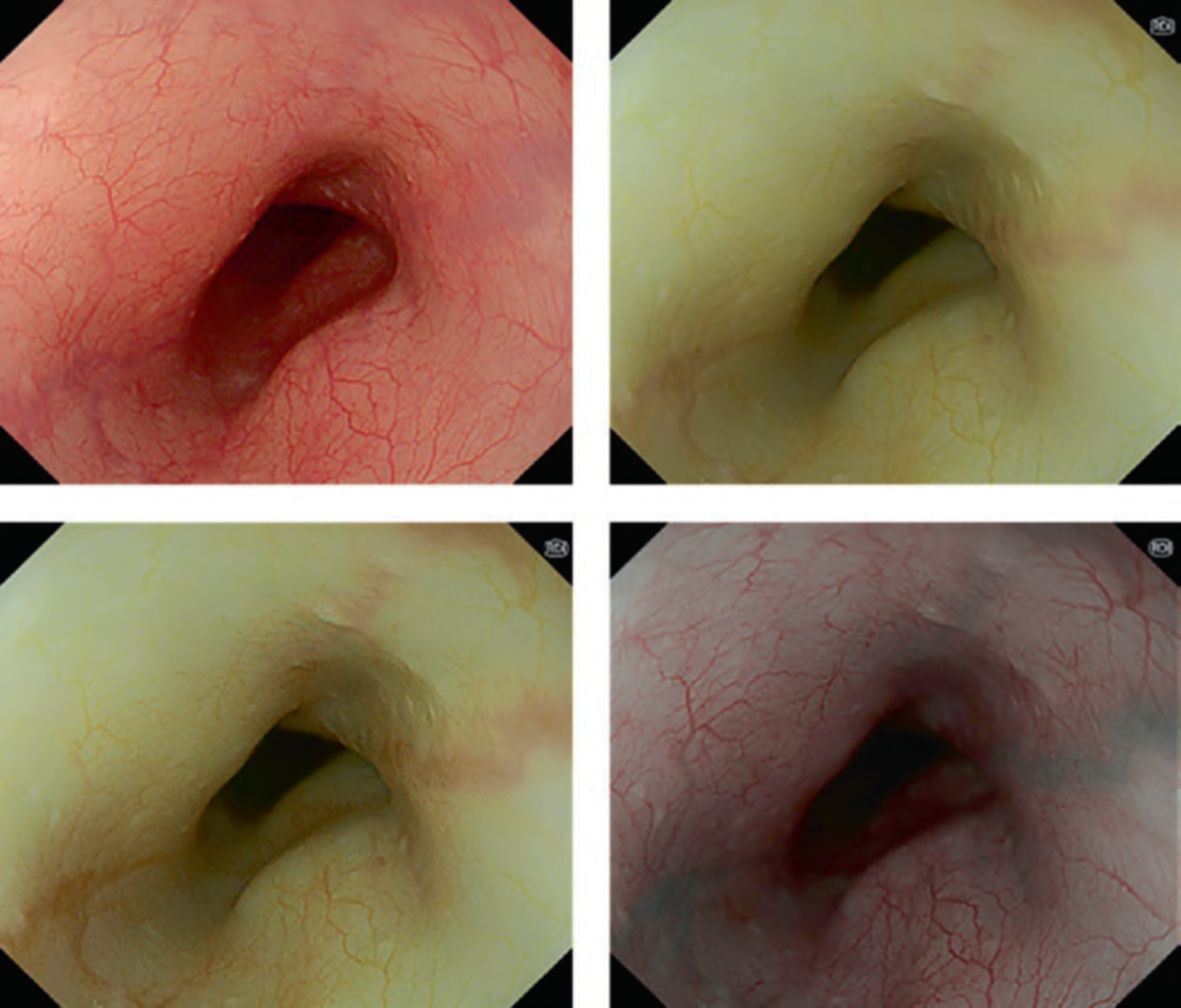
The flat pattern is a unique mucosal surface in BE showing an invisible/absent mucosal pattern, corresponding not to dysplastic but to non-dysplastic histology. The flat pattern was originally defined as invisible mucosal pattern (i.e., absence of pits and villi) with normal-appearing, long branching vessels. The flat pattern mimics an absent micro-surface (mucosal) pattern, which is significantly suggestive of early gastric cancer. As it was difficult for clinicians working in areas with a high incidence of gastric cancer to rate the flat pattern as non-dysplastic, recent studies proposed modified criteria for the “flat pattern.” This was to enhance the diagnostic accuracy for non-dysplastic lesions. This study used the modified criteria; according to these criteria, an invisible mucosal pattern without a distinct demarcation line and visible vascular patterns of long branching vessels or greenish thick vessels (GTV) was rated as “regular.” Thus, a flat pattern was rated as “regular” and the predicted histology as “non-dysplastic.” . Figure 3 shows representative NBI-M images of the flat pattern according to the modified criteria.
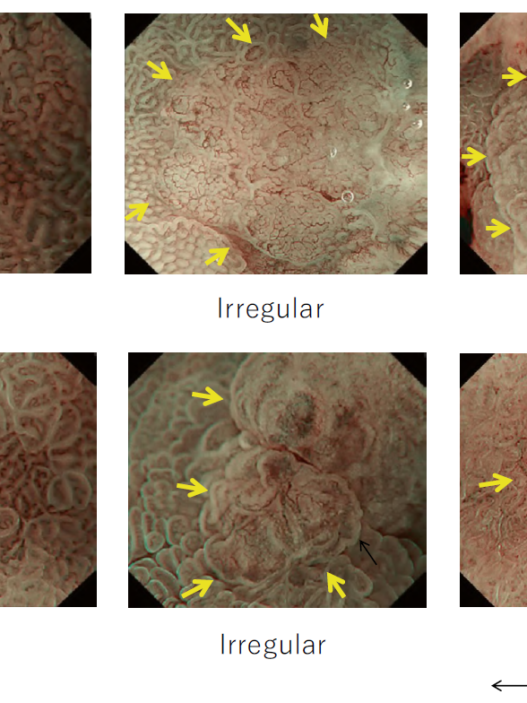
By using the diagnostic flowchart of the JES-BE classification (. Fig. 1), first, the mucosal pattern was classified as “visible” or “invisible” and rated as “regular” or “irregular” based on the diagnostic criteria for irregularity, as reported previously. The “invisible” mucosal pattern cannot be rated. Second, the vascular pattern was classified as “visible” or “invisible.” The “visible” vascular pattern included normal- appearing, long branching vessels and GTV previously reported and after- mentioned. General diagnosis was rated as “regular” or “irregular” based on mucosal plus vascular patterns. Finally, histology (“non-dysplastic” vs. “dysplastic”) was predicted according to the general diagnosis. “Dysplastic” corresponds to SBERN, including low-grade dysplasia (LGD), high-grade dysplasia (HGD), and superficial adenocarcinoma. Representative NBI-M images are shown in Fig. 4.
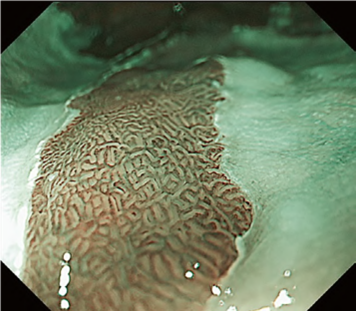
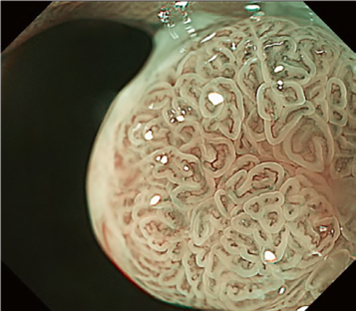
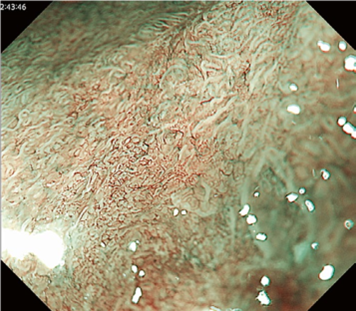
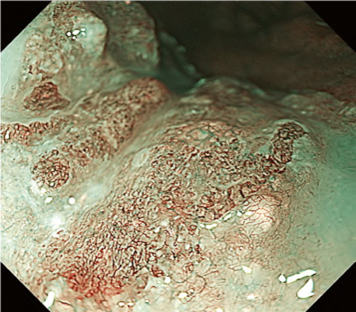
A nationwide multicenter study demonstrated the sensitivity and specificity for 10 reviewers (5 experts and 5 non-experts) were 87% and 97%, respectively. Overall accuracy, positive predictive value, and negative predictive value were 91%, 98%, and 83%, respectively. The overall strength of inter-observer and intra-observer agreements for dysplastic histology prediction was κ = 0.77 and κ = 0.83, respectively. No significant difference in diagnostic accuracy and reproducibility between experts and non-experts was found. The JES-BE classification system, including the diagnostic flowchart for predicting dysplastic BE, is acceptable and reliable, regardless of the clinician’s experience level.