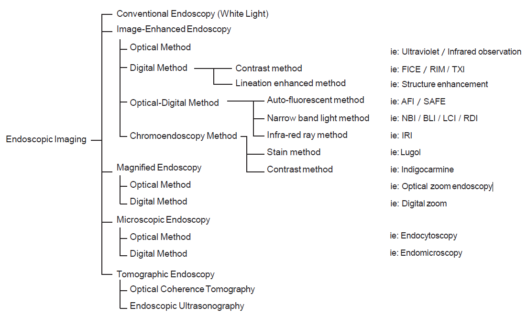
1 Introduction
Artificial Intelligence (AI) has ushered in an era of exciting new technology that is evident in numerous domains, including healthcare. One significant focus has been the integration of AI in medical imaging. In particular, systems referred to as computer- aided detection and diagnosis (CADe and CADx) have demonstrated considerable promise in the realm of gastrointestinal endoscopy. These systems employ AI to aid endoscopists in identifying abnormal regions and differentiating detected abnormalities, and this may contribute to improved patient outcomes.
One specific procedure in which these systems could prove highly beneficial is colonoscopy, a crucial tool for colorectal cancer screening. The practice of colonoscopy presents known limitations. Approximately one-fourth of colorectal neoplasms are missed during a single colonoscopy procedure [1], and the differentiation between neoplasms and nonneoplasms frequently falls below expected thresholds [2]. Several factors can influence the quality of colonoscopies, including the physician’s experience and the quality of bowel preparation, among others. AI-assisted systems employing deep learning may enhance the quality of the procedure by facilitating the detection and diagnosis of colorectal polyps.
While these systems appear to offer numerous benefits, it is imperative to rigorously analyze their performance in real-world settings. In this section, we endeavor to provide an overview of current research trends in AI applications for colonoscopy. We will also investigate how these systems have enhanced clinical outcomes and explore emerging concepts such as computer-aided quality (CAQ) improvement. By examining these topics, we aim to contribute to the growing body of knowledge regarding the utilization of AI in colonoscopy and suggest potential future directions.
2 Research Trends in CADe
In the field of colonoscopy, overlooking lesions, such as polyps, has long posed a significant challenge [1]; consequently, there was a desire for the development of CADe systems to detect polyps. Since the 2000s, various image feature parameters, such as edge detection and texture analysis, have been examined for integration into machine learning processes [3], primarily within the domain of information engineering for CADe of polyps. However, the accuracy of these CADe systems never stably exceeded 90%, with no instances of successful real-t ime detection assistance due to computational limitations.
Nevertheless, the advent of deep learning in the 2010s revolutionized the situation, with accuracy levels exceeding 90% and the emergence of the potential for real- time detection. In 2018, Misawa [4], Urban [5], and Wang [6] sequentially reported in medical journals the use of deep learning for real-time CADe systems. This highlighted the possible clinical applications of CADe in colonoscopy and spurred further fervent research and development. As of July 2023, in Japan, commercial distribution has commenced for products such as EndoBRAIN®-EYE (developed by Showa University and Nagoya University), Wise Vision (developed by NEC
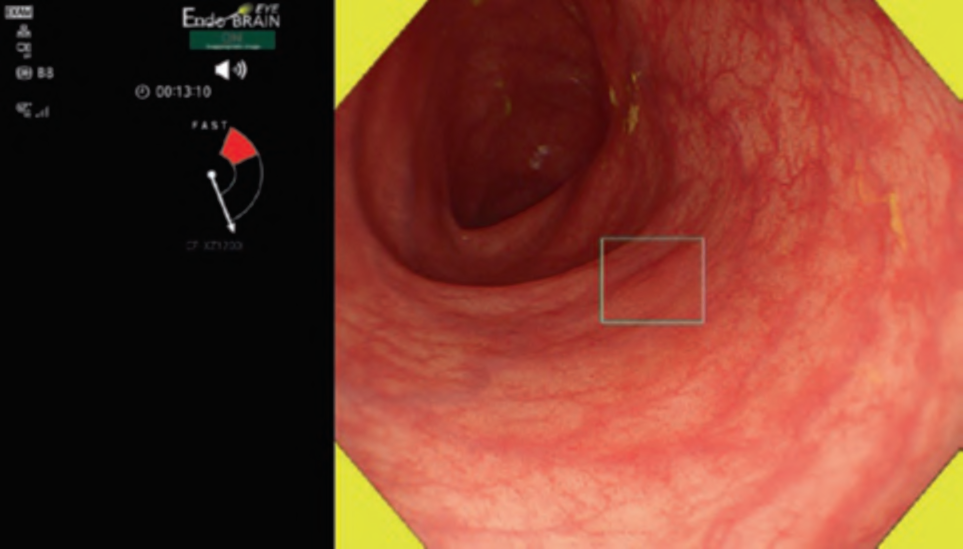
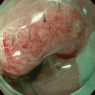
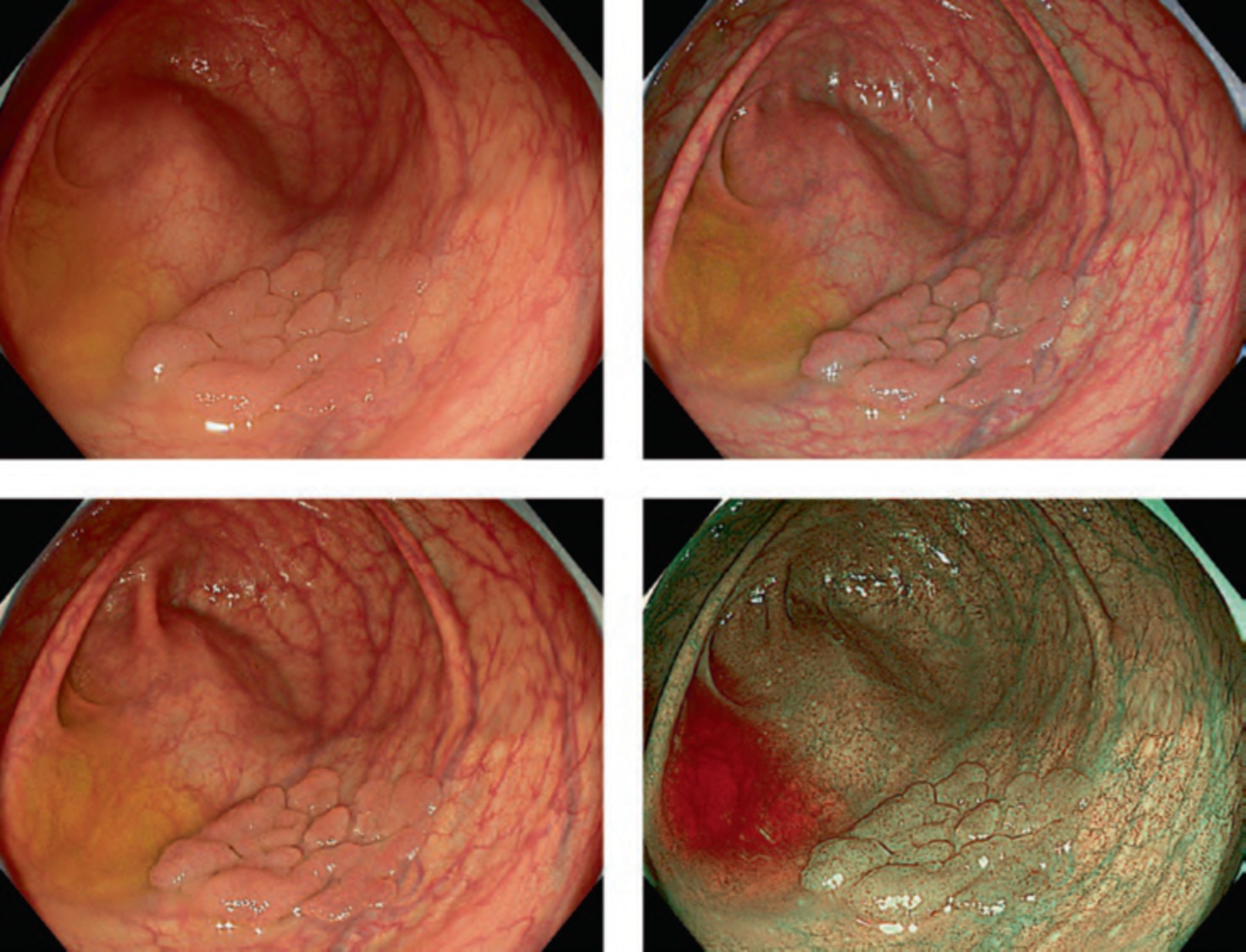
Corporation and the National Cancer Center), CAD EYE by Fujifilm Corporation, and EIRL Colon Polyp by L-pixel, all of which have received approval from the Japanese regulatory authorities. . Figure 1 shows an example of the clinical use of the EndoBRAIN-EYE. Looking around the world, numerous other products have been released. Within the scope of our research, several products have been launched on the market as CADe systems, including GI Genius (Medtronic), DISCOVERY (Pentax), Endo-AID (Olympus), MAGENTIQ-COLO (Magentiq EYE), CADDIE (Ordin Vision), EndoScreener (Wision AI), and Skout (ITERATIVE). Among these, GI Genius, EndoScreener, and Skout have become commercially available following approval from the US FDA.
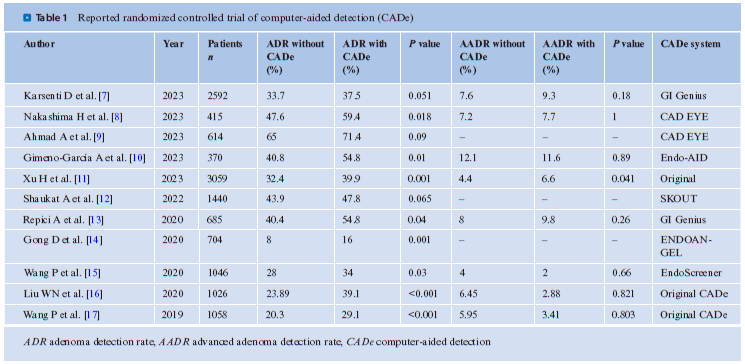
To what extent do these CADe systems improve clinical outcomes? . Table 1 summarizes the reported randomized controlled trials (RCTs) of CADe. In this context, RCTs refer to a study design wherein one group undergoes standard colonoscopy, while another is subjected to colonoscopy with CADe, implying a non-back-to-back comparison. With regard to the adenoma detection rate (ADR), in all but two trials, the utilization of CADe has been observed to significantly increase the ADR. A meta-analysis of CADe has demonstrated that its application can enhance the ADR by approximately 10% [18]. However, this meta-analysis also indicated that the polyps with increased detection rates due to CADe were predominantly diminutive adenomas (under 5 mm in size) and CADe did not contribute to the detection of advanced adenomas.
However, a recent large-scale RCT conducted by Xu et al. revealed a slight but statistically significant improvement in the detection of advanced adenomas within the CADe group [11]. These findings suggest that it may be premature to conclude that CADe is solely effective for diminutive adenomas. Furthermore, there may be potential biases in these studies, including but not limited to CADe. An example is the Hawthorne effect, where the examiner in the intervention group alters their behavior to improve the outcome. This bias may be present in reports from research groups involved in AI development or those with conflicts of interest with AI development companies. Consequently, large-scale prospective clinical studies, independent of AI developers, are deemed necessary to accurately ascertain the value of CADe.
3 Trends in the Research of CADx
In routine clinical practice, the ability to differentiate whether a detected lesion is neoplastic and, if so, whether it is benign or malignant, along with the determination of suitable surgical or endoscopic treatment, requires a high accuracy. However, achieving an accurate differential diagnosis can be challenging without substantial training. Therefore, support from CADx may prove valuable in enhancing diagnostic precision within this field.
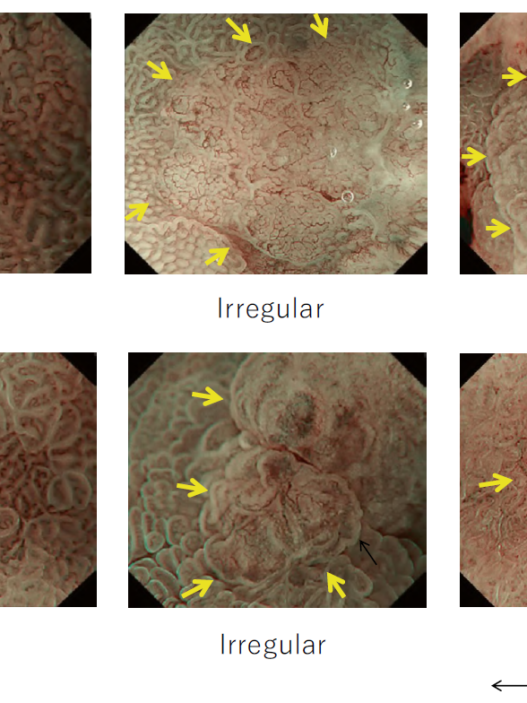
Research on CADx within the field of colonoscopy has been active since the 2010s. Multiple research groups have reported studies targeting vessels and surface patterns obtained from magnifying narrow-band imaging (NBI). In this research field, a group from Hiroshima University has been a pioneer. In a trial conducted by Kominami et al., CADx was employed to analyze 118 lesions in 41 patients, achieving a sensitivity and specificity of 93.3% for neoplasms, as reported in 2016 [19]. Subsequently, researchers Chen [20] and Byrne [21] reported the utilization of deep learning in CADx, with both studies achieving over 90% sensitivity in differentiating neoplasms from nonneoplasms. Mori et al. conducted a prospective study using EndoBRAIN, a CADx system designed for ultra-magnifying endoscopy, and reported that neoplasms could be differentiated with a sensitivity of 92.7% and specificity of 89.8%. This study underscores the potential utility of CADx in real-world clinical practice [22].
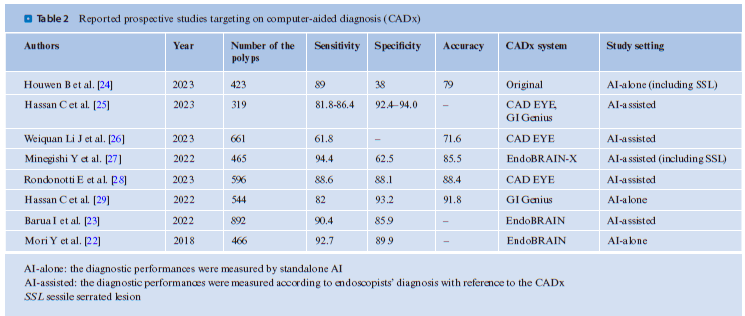
EndoBRAIN has been reevaluated in international multicenter studies in Japan, Norway, and the UK. Specifically, this study only included trainee endoscopists, who are expected to benefit most from AI. While the use of CADx did not lead to a significant increase in sensitivity (88.4% vs. 90.4%), it did result in a significant improvement in the proportion of high-confidence diagnoses (74.2% vs. 92.6%) [23]. In 2021, Fujifilm Corporation launched CAD EYE, a system capable of differentiating between neoplasms and nonneoplasms. CADx systems that classify neoplasms and nonneoplasms are gradually becoming integrated into daily clinical practice. . Table 2 lists prospective studies using CADx, as obtained by searching PubMed. As of July 2023, no RCT has been conducted, leading to a lower level of evidence. However, reports indicate relatively high diagnostic accuracy in real-world clinical practice. It should be noted that many of these reports emanate from AI developers or groups with conflicts of interest, warranting caution regarding the potential for bias.
4 Other Developments in CADx
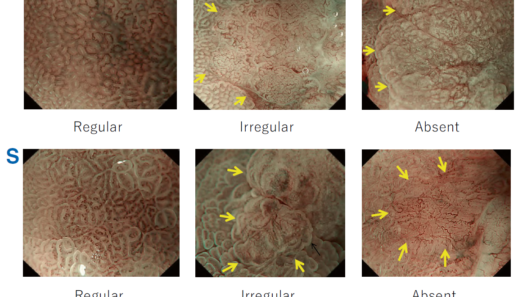
Early versions of CADx were primarily designed to assist in differentiating between neoplasms and nonneoplasms. However, as AI development technology has advanced, new targets for CADx are being eagerly investigated. These include the depth prediction of early colorectal cancer and differentiation between sessile serrated lesions (SSLs) and hyperplastic polyps. In real-world clinical practice, SSLs recently treated as a type of neoplastic polyp are gaining attention. Diagnosing the depth of a cancer is acknowledged to be challenging. Consequently, there is an increasing focus on more sophisticated CADx systems, moving beyond simple differentiation between neoplasms and nonneoplasms.
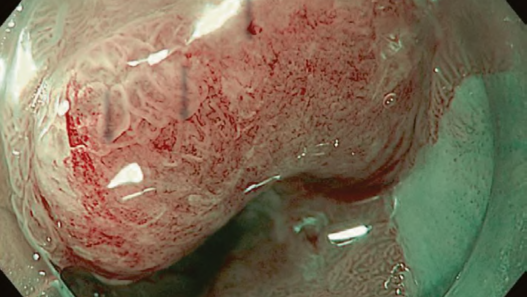
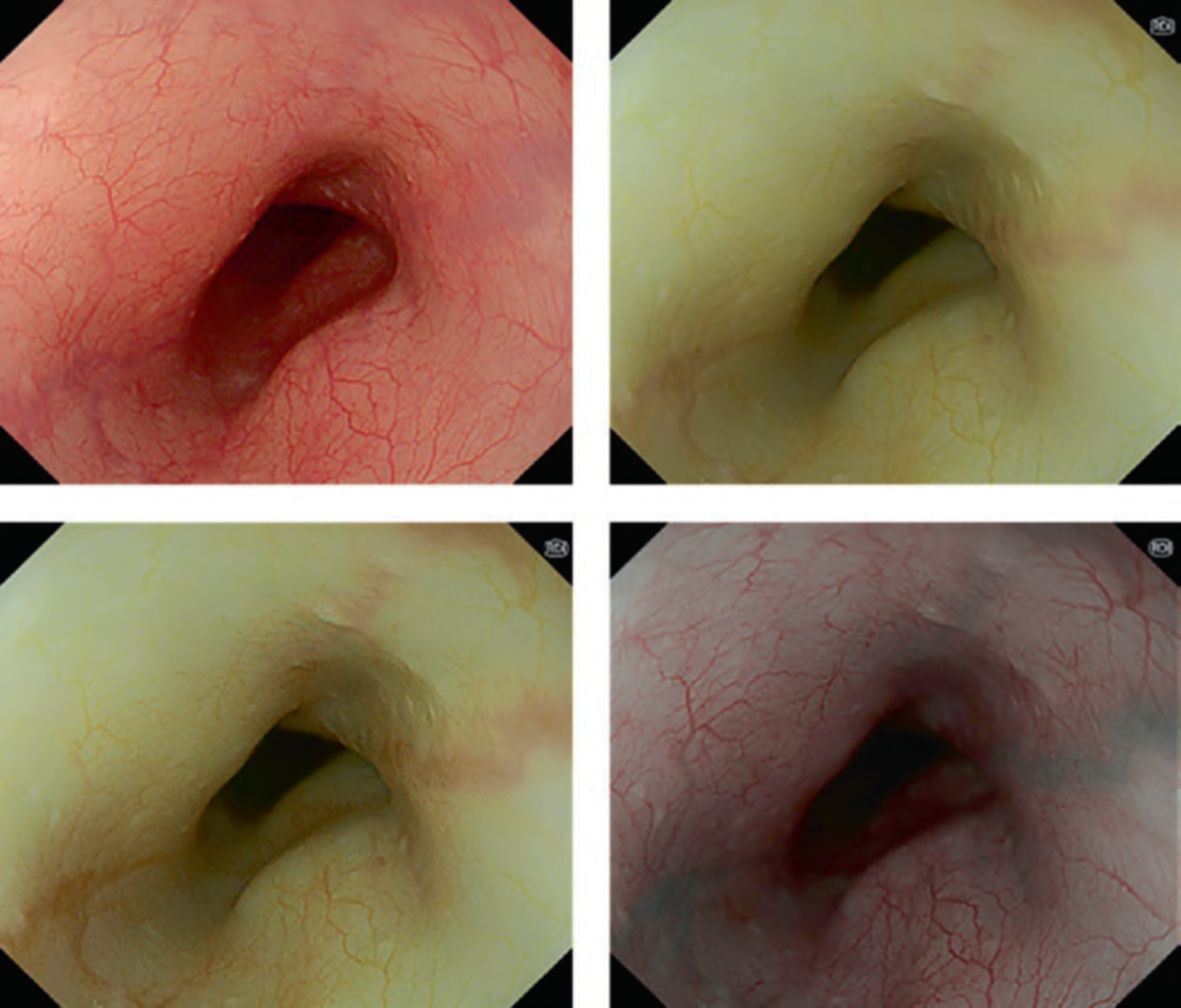
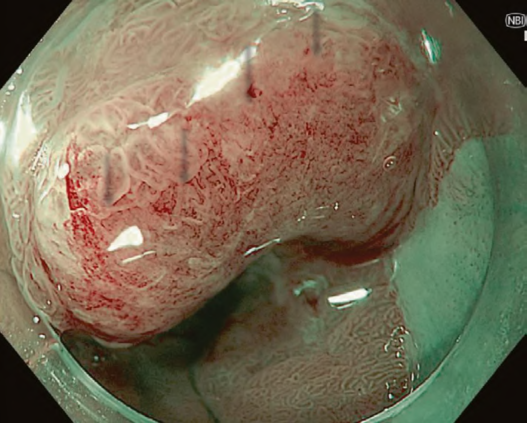
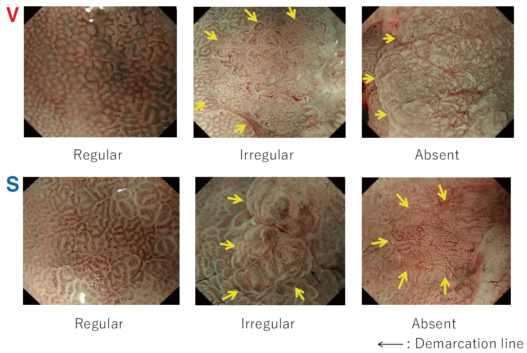
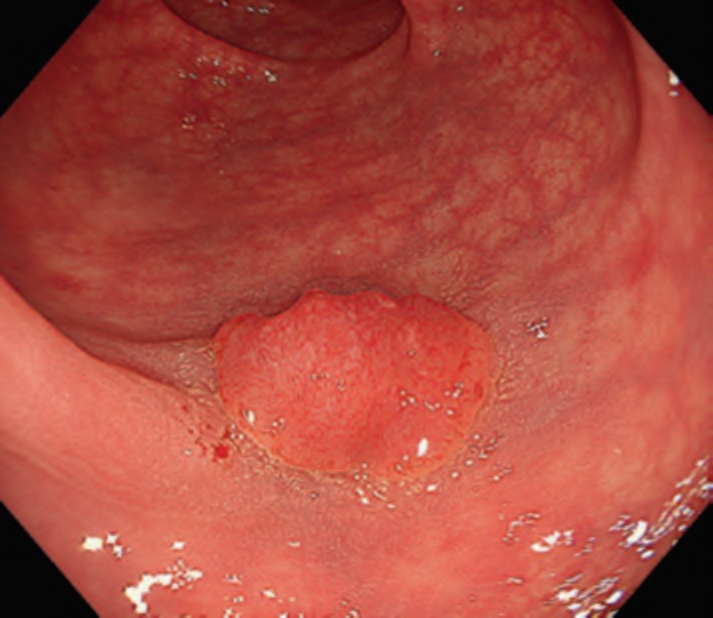
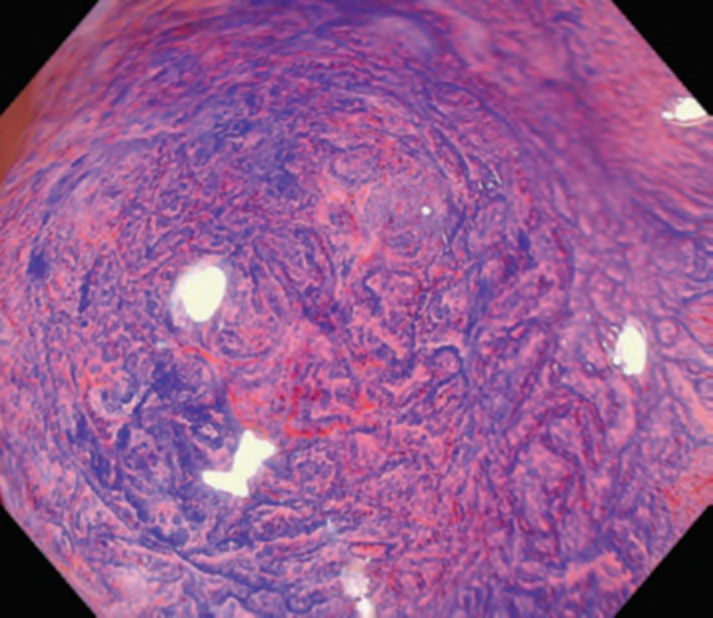
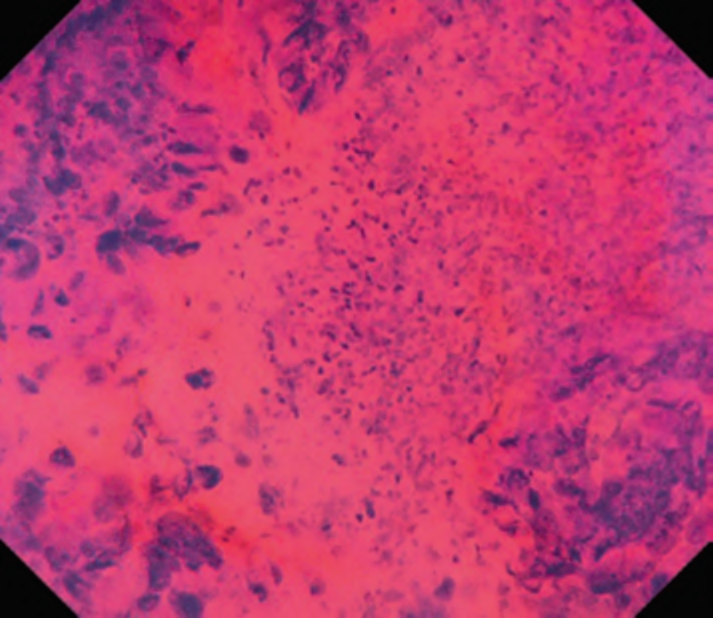
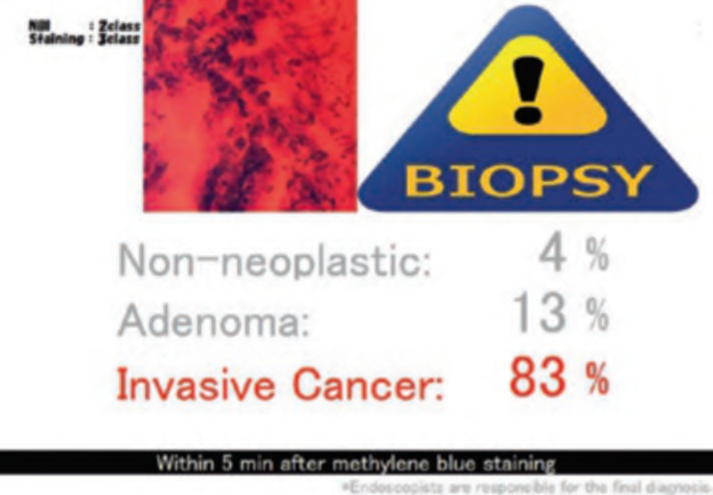
First, research on AI for the diagnosis of cancer depth has taken precedence, given its greater clinical importance. Tamai et al. developed a CADx for diagnosing invasive cancers (requiring surgical resection) using magnifying NBI images, reporting a sensitivity of 83.9% and specificity of 82.6% [30]. Takeda et al. also reported a correct diagnosis rate of 94.1% for invasive cancers using CADx for endocytoscopy (EndoBRAIN-Plus) [31]. . Figure 2 shows an early-stage colorectal cancer (T1) that diagnosed using the EndoBRAIN-Plus. Tokunaga et al. created a more versatile and practical CADx system, capable of determining whether the target lesion can be treated endoscopically, based on white light images [32]. This system was retrospectively verified and reported an accuracy of 90.3%. Okamoto et al. developed a CADx to identify JNET classification types 1, 2A, 2B, and 3, achieving an accuracy of 94.1% for JNET type 3 [33].
Minegishi et al. conducted a prospective study using a CADx system capable of identifying SSL. According to their study, the sensitivity for neoplastic lesions, including SSL, was 94.4%, while the specificity was 62.5% [27]. This CADx, named EndoBAIN-X, obtained approval from the Japanese regulatory body. Houwen et al. are developing a similar CADx system and conducting prospective clinical studies, and the sensitivity of their CADx system for tumorous lesions, including SSL, was 89%, while the specificity was 38% [24]. Consequently, clinical studies of CADx systems treating SSL as neoplasms are being gradually reported.
5 Other Applications of AI
It has been observed that the usage of AI alone may not adequately enhance clinical outcomes. Recent negative studies evaluating the performance of CADe have been reported [34]. This has led to the emergence of, and growing acceptance for, a novel concept known as CAQ. The ability of CADe to detect lesions is limited to instances in which lesions, such as polyps or cancers, are visually depicted in endoscopic monitor. For instance, if polyps or cancers are located in blind spots, such as folds, residual feces, or behind flexures, CADe is found to be ineffective. This limitation arises because CADe, being solely an image analysis technology, is only effective for lesions that are visualized on the monitor.
It is hypothesized that the development of a system to guide colonoscopy with fewer blind spots could work in conjunction with CADe to further reduce missed lesions. In this context, Yao and his team developed a system that monitors the withdrawal speed of endoscope. The system issues real-time alarm if the withdrawal is excessively fast. The use of this system significantly improved the lesion detection rate, as reported by Yao et al. [35]. The monitoring of withdrawal speed likely encouraged more careful observation of the colonic mucosa, thus altering endoscopists’ practices.
6 Conclusions
The introduction of deep learning has democratized AI technology, enabling the rapid development of high-accuracy AI systems. This breakthrough has accelerated the clinical implementation of AI, making it an integral part of many medical procedures, including endoscopy.
Every day, numerous new studies on endoscopic AI are reported, adding to our understanding of AI’s potential and limitations in this field. Some AI systems, including EndoBRAIN, have already received regulatory approval and are being implemented in clinical settings.
However, while AI has shown tremendous potential in enhancing detection and diagnostic capabilities in colonoscopy, it is essential to remember that the long-term benefits of AI usage, such as reducing cancer incidence and mortality, remain unproven. Therefore, moving forward, the focus should be on conducting large-scale prospective studies to verify these benefits and assess the overall utility of AI in endoscopy. The exploration of AI’s role in medicine is only beginning, and the horizon promises many more exciting discoveries.